Flange Breakage Resistance
ISO 11040-4 Annex C1
Flange breakage resistance is a critical quality attribute in prefilled syringe manufacturing. This test determines the mechanical strength of the syringe barrel’s flange—the portion that must withstand applied forces during clinical use. As healthcare regulations tighten globally, meeting the mechanical performance standards outlined in ISO 11040-4 Annex C1 is essential for manufacturers of glass syringes and sterilized subassembled syringes.
Cell Instruments, a leader in precision testing equipment, offers robust solutions to ensure that your syringe testing complies with international standards while maximizing accuracy and operational efficiency.
ISO 11040-4 and Its Application in Syringe Testing
ISO 11040-4 governs glass barrels for injectables, particularly focusing on structural integrity and functionality. In ISO 11040-4 Annex C1, specific guidelines are laid out for evaluating flange breakage resistance. The goal of this test is to simulate conditions under which a syringe’s flange might break during handling or automated filling processes.
The standard applies not only to final syringe barrels but also to sterilized subassembled syringes ready for filling, ensuring consistency across the supply chain.
Why Flange Breakage Resistance Testing Matters
Syringes undergo numerous mechanical stresses during transport, storage, filling, and administration. The flange, a structural component at the end of the syringe barrel, is a known weak point. If the flange fails, it can compromise the sterility, dosage accuracy, and ultimately the patient’s safety.
Accurate flange breakage resistance testing helps:
- Validate design and manufacturing processes
- Meet regulatory compliance
- Reduce product failure risk in the field
- Build trust with regulatory bodies and clients
ISO 11040-4 Annex C1 Flange Breakage Resistance Test Method
Flange Breakage Resistance Tester Required
The method outlined in ISO 11040-4 Annex C1 consists of applying a controlled compressive force to the syringe barrel flange until it fails. Here’s how it’s done:
Universal tensile and compression testing machine (e.g., Cell Instruments’ Syringe Tester)
Syringe holder made of suitable material like PEEK
Loading pin, preferably polyacetale with stainless steel core

ISO 11040-4 Annex C1 Test Procedure
1. Prepare Test Samples
Avoid pre-testing shocks. Visually inspect each syringe and holder for damage.
2. Testing Machine Setup
Place the syringe barrel into the holder. Align the loading pin near the syringe’s shoulder as per Figure C.2 of the ISO standard.
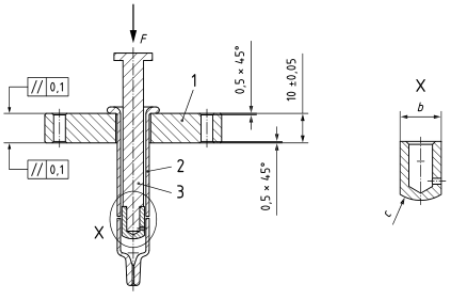
3. Testing Execution
- Set the machine speed to 100 mm/min (or per agreement).
- Initiate the test and apply force until flange breakage occurs.
- Record force vs. displacement to create a curve.
4. Data Analysis
Identify the peak force from the curve—this value represents the flange breakage resistance.
5. Reporting
Include test speed, number of samples, peak values, and any anomalies. Ensure documentation meets ISO traceability requirements.
Syringe Tester from Cell Instruments
Syringe Testing Machine Key Advantages
- Compatible with syringe-specific fixtures
- Real-time graph plotting of force-displacement
- Adjustable speeds and load ranges
- Built-in microprinter and optional RS232 port for seamless data export
The system’s modularity makes it ideal for companies engaged in comprehensive syringe testing, including flange breakage resistance and other mechanical evaluations.
Syringe Testing Machine Best Practices
- Use high-frequency sampling (≥500 Hz) for peak accuracy
- Verify test alignment before each run to avoid false readings
- Change worn components (loading pin, holder) regularly to maintain consistency
- Document everything, even minor deviations, to enhance traceability
- Collaborate with customers to agree on test speeds and equipment calibration
Flange breakage resistance testing is not just a compliance checkbox—it’s a vital part of product quality assurance for syringe manufacturers. By aligning testing practices with ISO 11040-4 Annex C1, you can ensure product durability, safety, and regulatory acceptance.
Cell Instruments is your trusted partner in achieving testing excellence. Our Syringe Tester helps streamline syringe testing workflows while delivering the accuracy and compliance demanded by modern healthcare standards.
Let us support your journey toward high-performance, regulation-compliant products.