Syringe Glide Force Testing
ISO 11040-4 Annex E
In the medical and pharmaceutical industries, ensuring the performance and quality of syringes is paramount. One critical method for assessing syringe functionality is through the ISO 11040-4 syringe glide force testing. This standard helps manufacturers ensure that syringes, whether lubricated or non-lubricated, perform consistently and reliably when used for drug delivery.
What is ISO 11040-4 Syringe Glide Force Testing?
ISO 11040-4 outlines the procedure for measuring the glide force of a syringe, which is the force required to move the plunger within the barrel. This test is vital for assessing the quality and consistency of syringes, especially when they are used in medical applications.
The purpose of this testing is twofold:
- Lubrication Assessment: It helps determine the effectiveness of the lubrication inside the syringe barrel, usually achieved with silicone oil or other materials.
- Non-Lubricated Syringes: The test also evaluates non-lubricated syringes, including those filled with water for injection, simulating actual usage conditions.
This procedure ensures that syringes meet certain glide force standards, reducing friction during injection and ensuring patient safety and comfort.
The Importance of Glide Force in Syringes
The glide force test is not just about ensuring smooth movement of the plunger. The glide force indicates the performance quality of the syringe:
- High Glide Force: Excessive friction may lead to discomfort during injection and could even cause difficulty for healthcare professionals in administering the medication.
- Low Glide Force: Insufficient friction may result in leakage, making the syringe unreliable and potentially unsafe for medical use.
Materials Required for ISO 11040-4 Syringe Glide Force Testing
For effective testing, certain materials and equipment are necessary, including:
- Sterilized Syringes: The syringes tested should be ready for filling, with proper sterilization to avoid contamination.
- Plunger Stoppers: These should be compatible with the syringe barrel and its intended use.
- Plunger Rods: These are essential for applying the necessary force during testing.
These materials need to be agreed upon between the manufacturer and the customer, with specific attention given to the type of plunger stopper and its lubrication.
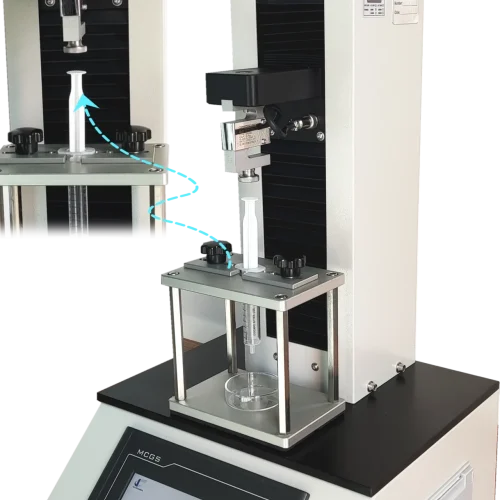
Apparatus Used in ISO 11040-4 Testing
The ISO 11040-4 testing requires specialized equipment:
- Universal Tensile and Compression Testing Machine: This machine is used to apply the force at a specific speed, typically 100 mm/min, to measure the glide force accurately. The force range should go up to at least 50 N, depending on the syringe’s specifications.
- Syringe Support and Adaptor Plates: These are used to hold the syringe in place during the test, ensuring that the force is applied correctly.
- Vent Tube or Vacuum Stoppering Tool: This tool is used to install the plunger stopper into the syringe barrel, maintaining sterility and proper positioning.
Procedure for Performing the Test
The ISO 11040-4 glide force test follows a systematic procedure:
- Insert the Plunger Stopper: Place the plunger stopper in the syringe barrel using either a vent tube or vacuum stoppering method.
- Set Up the Plunger Rod: Attach the plunger rod to the stopper, ensuring it aligns with the testing device.
- Place the Syringe in the Testing Device: Position the syringe in the adaptor plate of the tensile testing machine.
- Apply Force: Start the compression test, applying force at the agreed speed, typically 100 mm/min.
- Measure Maximum Force: Record the maximum force experienced in the glide force test region, which is the section between the initial force and the sharp increase at the end of the plunger stroke.
- Repeat for Multiple Samples: Perform the test for several syringes to ensure consistency.
Syringe Plunger Force Calculation
The results of the test are documented, including:
- Maximum Gliding Force: The highest force observed in the glide force test region.
- Average Gliding Force: The calculated average force, which provides an overall picture of the syringe’s performance.
- Test Documentation: Any deviations or observations should be noted for traceability and compliance.
Key Considerations for Accurate Glide Force Testing
- Test Speed: A speed of 100 mm/min is typically used, but this can vary depending on the manufacturer’s requirements.
- Sampling Rate: A higher sampling rate (e.g., 500 Hz) is recommended for more accurate peak measurements.
- Test Environment: The testing environment should be controlled to avoid external factors influencing the results, such as temperature or humidity.